1.Quality requirements
According to general principles of pharmacopoeia:
Accurate content and narrow weight distribution;
Proper hardness;
Uniform color, integration and beauty;
Disintegration time and dissolution rate as required;
In accordance with requirement on uniform content for small-dosage medicines or medicines of strong efficacy;
In accordance with requirements on hygiene inspection.
2.Common excipients
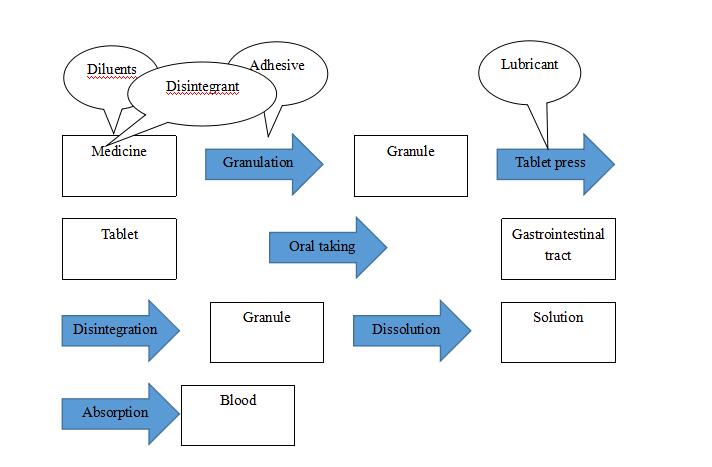
1)Excipients
They are additional materials in medicine but not API and have no physiological activity, also known as adjuvants.
Common excipients:
Diluents: to increase weight or volume;
Adhesive: to agglomerate;
Disintegrant: to break tablets into small granules;
Lubricant: for fluent flowing, no sticking, lubrication;
Other excipients: color/smell/taste conditioner, absorption enhancer.
2)Dilunets
Also known as filler, to increase weight or volume;
Starch
Sucrose
Dextrin
Formula of blank tablets:
Starch: 53Kg
Sugar: 20Kg
Dextrin: 3Kg
10% starch for granulation
1% magnesium stearate
Dosage of dextrin should be small
Lactose:
Non hygroscopicity
Good compressibility
Tablets containing it are smooth and beautiful
Spray-dried lactose
Produced through spray drying method
Granules are spherical
Good fluidity and compressibility
Used in direct tablet press for powders
Pregelatinized starch
Good fluidity and compressibility
Self-lubricity and dry adhesion
Good disintergration
Microcrystalline cellulose (MCC)
Strong adhesion and compressibility
Well-known as dry adhesive
Goods name is Avicel abroad
Inorganic salts
CaSO4, CaHPO3, CaCO3.
CaSO42(H2O) is common.
CaHPO4·2H2O can be used in direct tablet press for powder.
Tablets containing them are of smooth appearance, proper hardness and good disintegration.
They have no absorption on medicines.
Sugar alcohols
They are commonly used for chewable tablets.
Mannitol and sorbitol.
For cool feeling.
3)Moinstening agent
Liquids have no viscosity but can make materials to be viscous so that granulation is promoted.
Distilled water
Ethanol (30-70%)
4)Adhesives
They can make materials of no viscosity or weak viscosity to be viscous so that materials can be agglomerated.
Starch
common concentration is 8%-15, and 10% is the commonest.
Starch flashing: Starch is suspended into water (once or 1.5 times more than starch). Then certain volume of boiling water is flashed according to concentration requirements. The solution is continuously mixed and gelatinized;
Starch boiling: Starch is suspended into water of enough volume in a jacketed container. The container is heated and the solution is stirred until starch is gelatinized. It’s better to not use straight fire in order to avoid coking.
Cellulose derivative
Methylcellulose (MC): soluble in water and insoluble in alcohol;
Hypromellose (HPC): soluble in water and alcohol;
hydroxypropyl methylcellulose (HPMC): soluble in water but not in alcohol;
Sodium carboxymethyl cellulose (CMC-Na): soluble in water but not in alcohol;
Ethyl cellulose (EC): soluble in alcohol but not in water.
Povidone (PVP)
Common specification is K30 with molecular weight of 50,000;
It is soluble in water and ethanol and the solution is viscous;
PVP-ethanol solution is used for medicines sensitive to humidity and temperature;
Dry adhesive for tablet press of powders;
For effervescent tablets, 5% PVP - absolute ethanol is used to avoid chemical reaction between acid and alkali during granulation;
Good adhesive for chewable tablets;
Shortage: good hygroscopicity.
Gelating
Granulation should process in high temperature to avoid gelatinization;
One shortage is that materials will harden as time goes;
It is suitable for medicines which are loose or cannot be granulated easily, or some buccal tablets which needn’t disintegration or working in a certain time.
Others
Polyethylene glycol (PEG): PEG4000, PEG6000
Sucrose: 50-70%
Sodium alginate
5)Disintegrant
Definition: the excipients used for quick tablet disintegration to small granules. It is not added in sustained-released tablets, buccal tablets and chewable tablets.
Functions: to eliminate the binding force generated by adhesive or strong compression and to disintegrate tablets.
Capillary action
Swelling action
Heat of wetting
Gas production
Dry starch
Swelling rate: 186%
Classic disintegrant; capillary forming agent; containing less than 8% water;
It can be used for medicines which are insoluble or slightly soluble in water.
Sodium carboxymethyl starch (CMS-Na)
Swelling rate: 300 times
Low substituted hydroxypropyl cellulose (L-HPC)
Swelling rate: 500%~700%
Being of large surface area and porosity and widely used in China
Croscarmellose sodium (CCNa)
Swelling rate: 400%~800%
Used with CMS-Na for better disintegration
Crospovidone (PVPP)
Water absorption: 60%
Active capillary and good hydration
Effervescent disintegrant
Specially used for effervescent tablets;
Sodium bicarbonate + organic acid;
Organic acid: citric acid, tartaric acid, fumaric acid;
Properly packed to avoid effect loss due to moisture
Adding method
Adding after granulation: Disintegrant is added into dry granules before tablet press and disintegration takes place among granules;
Adding during granulation: Disintegrant is added during granulation and disintegration takes place inside granules.
Adding both after and during granulation: Part of disintegrant is added during granulation and the other part after granulation. Disintegration takes place both inside and among granules for good disintegration. Generally, 50~75% disintegrant is added during granulation and 25%~50% is added after granulation.
Disintegration speed: adding after granulation>adding both after and during granulation>adding during granulation.
Dissolution rate: adding both after and during granulation>adding during granulation>adding after granulation.
6)Lubricant
Glidant: for fluent material feeding;
Antiadherent: for less material sticking to punch;
Lubricant: to reduce friction and beautify and smooth tablets;
Proper lubricants should be added into materials before tablet press.
Glidant
It can better fluidity and filling status of granules and narrow weight distribution;
Aerosil: good gliant for direct tablet press. Its specific surface area is large. Common proportion is 0.1~0.3%.
Talc: It can make up depression on granule surface and better granule fluidity. Its specific surface area is large and common content is 0.1~0.3%.
Antiadherent
Excipients to prevent material from sticking to puncher and for fluent tablet pressing and smooth tablet surface.
Lubricant
It can reduce the friction between tablets and wall of punching mold so that tablets will not be broken.
Magnesium stearate: hydrophobic lubricant. 0.1%-1% is its proper content rate. Too much magnesium stearate will lead to slow disintegration or dissolution of tablets.
Hydrogenated vegetable oil: a good lubricant made by spraying drying method. It can dissolve in light liquid paraffin or hexane. They are sprayed on dry granules and then mixed.
PEG4000 and PEG6000
Sodium (magnesium) lauryl sulfate: soluble in water
7)Color, smell and taste conditioner
Colorant
Medicine grade
Dosage: <0.05%
Paying attention to reaction between colorant and API and color migration during drying.
Flavoring
Dissolved in ethanol first and sprayed on dry granules then;
Microencapsulated solid flavoring: stevioside, aspartame
3.Formula table
|
Formula |
Content |
Proportion |
|
Cimetidine (80 meshes) |
20 Kg |
56.50% |
|
Starch (100 meshes) |
8 Kg |
22.60% |
|
Starch slurry (10%) |
5 Kg |
14.12% |
|
Dry starch (100 meshes) |
1.7 Kg |
4.80% |
|
Talc (80 meshes) |
0.6 Kg |
1.69% |
|
Lamivudine |
200 |
|
Starch |
100 |
|
Microcrystalline cellulose |
170 |
|
L-HPC |
15 |
|
PVPK30 (10%) |
38.4 |
|
Carboxymethyl starch sodium (CMS) |
35 |
|
Magnesium stearate |
7.4 |
Aspirin
|
Starch |
2.0 Kg |
|
Lactose |
2.0 Kg |
|
NaHCO3 |
1.0 Kg |
|
Anhydrous citric acid or fumaric acid |
5.0 Kg |
|
Aspartan |
0.1 Kg |
|
SiO2 |
0.2 Kg |
|
Orange flavoring |
0.1 Kg |
|
Aspirin |
81.0 g |
|
Saccharin Sodium |
2.0 g |
|
Lactose |
120.0 g |
|
Microcrystalline cellulose |
158.0 g |
|
Mannitol |
40.0 g |
|
Acacia senegal |
4 g |
|
Aspartame |
50 mg |
|
Cefuroxime Ester |
3010 g |
|
Microcrystalline cellulose |
1550 g |
|
Crospovidone |
480 g |
|
SiO2 |
60 g |
|
Sodium dodecyl sulfate(SDS) |
60 g |
|
Stearic acid |
360 g |
|
Aspartame |
360 g |
|
Orange powder flavoring |
12 g |