1) Problems during tablet press
|
Problems |
Causes |
Solutions |
|
Tablet breaking |
Technique: single punch tablet press, quick tablet press, convex tablet
Formula: much fine powder, dry granule, poorly viscous or small-dosage adhesive, thick tablet |
Using excipients of small elasticity and large plasticity |
|
Loose tablet |
Improper adhesive or pressure |
|
|
Sticking to punch |
High water content, improper lubricant, rough puncher surface and high humidity |
|
|
Overrun of tablet weight difference |
Poor granule fluidity;
Fine powder or disparate size of granules;
Fluctuation of material amount in material feeding hopper;
Punching not matching mold hole well. |
Granulating again or using better glidant;
Removing fine powders or granulating again;
Granules taking up 1/3 of the hopper;
Changing puncher and mold ring. |
|
Slow disintegration |
Type and dosage of disintegrant, type and dosage of lubricant, viscosity and dosage of adhesive, pressure and hardness |
|
|
Dissolution overrun |
No disintegration of tablets; excessive hardness of granules, poor dissolution of medicine |
|
|
Non-uniform content |
For small dosage medicine: 1) non-uniform mixing; 2) migration of soluble ingredients among granules. |
Using insoluble colorant;
Applying microwave heating;
Overturning granules often during box drying |
2) Dry granulation
a. After uniform mixing, the mixture of drug powder and excipients is compressed to the solid with proper equipment. Then the solid are ground to dry granules of appropriate size. At last, these granules are pressed to tablets.
b. Methods: heavy compression and roller compaction
c. Advantages: Dry granulation tablet press is properly used for materials which are sensitive to temperature or decomposable in water; the method is easy , time and labor saving. But phenomena of crystal change or activity reduction should be noticed.
3) Direct powder tablet press
a. Drug and excipients are directly mixed and compressed to tablets without granulation.
b. Advantages
a) time and energy saving, simple process...
b) Applicable for materials sensitive to humidity and temperature
c. Shortage: high demand on powder fluidity
d. Advancement
a) Good medical excipients: MCC, compressible starch, sprayed dry lactose, Ca2HPO4·2H2O, micro-powdery silica gel...
b) Equipment advancement: powder feeder and vibrating device for compulsory powder feeding; additional powder & dust collecting device
8. Tablet coating
1) Goal
a. To prevent moisture, light and air to stabilize medicine;
b. To cover bad smell of medicine;
c. To beautify tablets;
d. To control medicine release, such as enteric-coated tablets, sustained and controlled-release tablets.
2) Type:
sugar-coated tablets & film-coated tablets
3) Techniques
|
Name |
Function |
Material |
Layer qty |
|
Isolation layer |
To isolate moisture |
Glue |
3-5 |
|
Powder-coated layer |
To remove edges and corners |
Sugar syrup |
3-5 |
|
Sugar-coated layer |
To smooth tablet surface |
Thick sugar syrup |
10-15 |
|
Colored sugar coat |
For identification and beauty |
Colored syrup |
8-12 |
|
Polishing layer |
To prevent moisture and smooth tablets |
Chinese wax |
2 |
4) Film coating technique and material
a. A layer polymer film is tightly coated on tablet core;
b. Advantages:
a) Compared with sugar coating, film coat features short production period, high efficiency, small dosage of material and strong moisture prevention.
b) Sustained release tablets, controlled release tablets and enteric tablets can be processed in this way.
c. 1954 saw the first vending of film coated tablets in America;
d. Water dispersion coating came out in 1970s;
e. Film coating is introduced into China in 1980s.
5) Film coating type
a. Organic solution coating: coating materials are dissolved in organic solvent (such as ethanol and acetone). Tablets are then coated through coating techniques;
b. Water dispersion coating: coating materials are suspended in water o that water dispersion is made (<1um) and can coat tablets through coating techniques.
6) Film coat
a. Gastrointestinal coat: HPMC, HPC, Methylmethacrylate Resin VI, Eudragit E, PVP;
b. Enteric coat: HPMCP, CAP, Polyacrylic Resin I, Polyacrylic Resin II, Polyacrylic Resin III, Eudragit L, Eudragit S;
c. Insoluble coat: EC, CA.
7) Coating liquid ingredients
a. Film coat: gastrointestinal coat, enteric coat and insoluble coat;
b. Plasticizer: propylene glycol, castor oil and dibutyl phthalate;
c. Porogen: sucrose, sodium chloride and PEG;
d. Sunscreen: titanium dioxide;
e. Solid material: talc;
f. Colorant: amaranth, carmine, lemon yellow, indigo;
g. Solvent: water, ethanol, acetone.
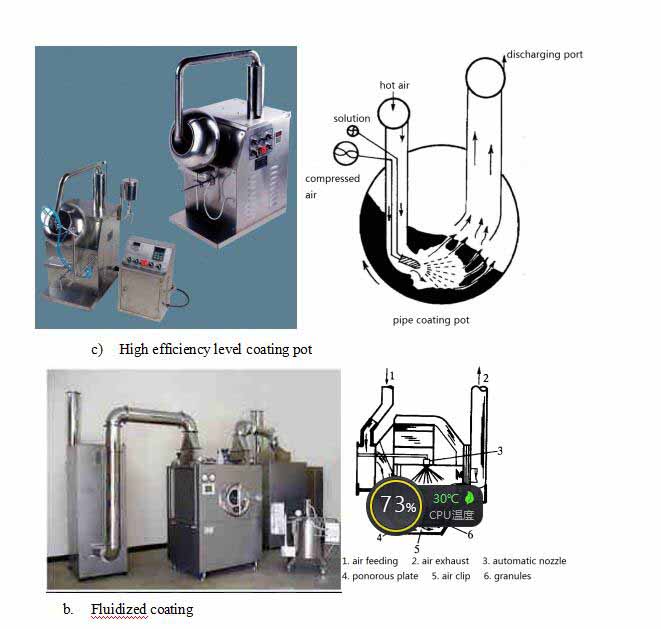
8) Coating method and equipment
a. Rotary coating: the most common;
a) Also known as pot coating
b) Tilting coating pot and pipe coating pot
c) High efficiency level coating pot
b. Fluidized coating
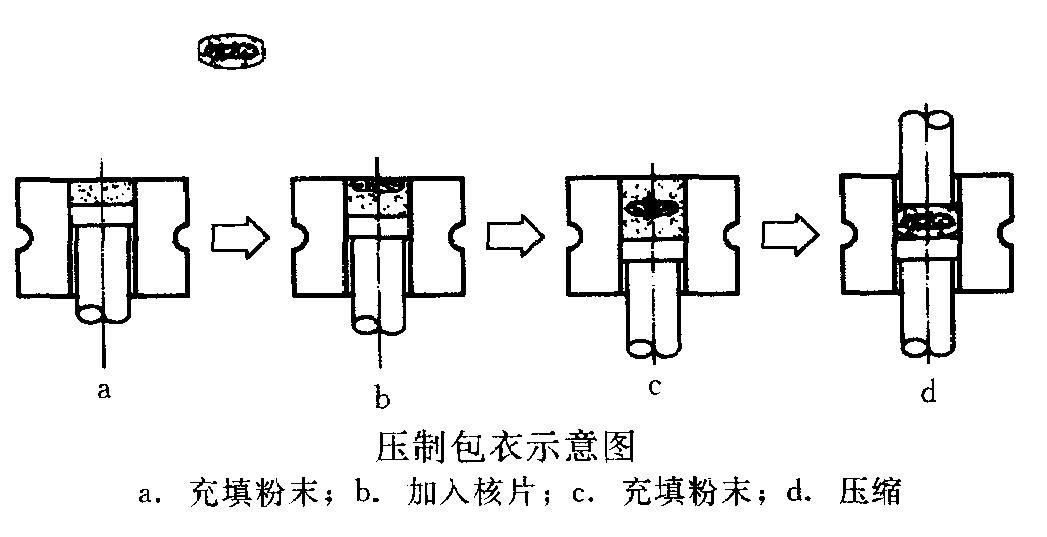
c. Compression coating
a) Bad influence of water and high temperature on medicine can be avoided;
b) Shot production time, high automation and good production conditions;
c) Shortage: high demand on accuracy of tablet press.
a. Powder filling; b. tablet core feeding; c. powder feeding; d. compression
9) Formula of enteric coating erythromycin tablet
|
Acrylic resin |
0.028Kg |
|
Castor oil |
0.0168Kg |
|
85% ethanol |
560ml |
|
Diethyl phthalate |
5.6Kg |
|
Polysorbate 80 |
5.6Kg |
|
Talc |
0.0168Kg |
10) Tablet quality testing
a. Appearance performance
b. Tablet weight distribution
a) <0.3g: ±7.5%
b) ≥0.3g: ±5%
c. Hardness and friability
a) Influencing tablet production, transport and storage
b) Influencing tablet disintegration and dissolution
d. Disintegration time
a) The following tablets needn’t disintegration testing: tablets needing dissolution testing regulated by pharmacopoeia, sustained and controlled release tablets and chewable tablets
e. Content uniformity
a) Medicine of small dosage (<25mg)
b) API being less than 25% of tablet weight
c) Distinguishing from content testing
f. Dissolution degree
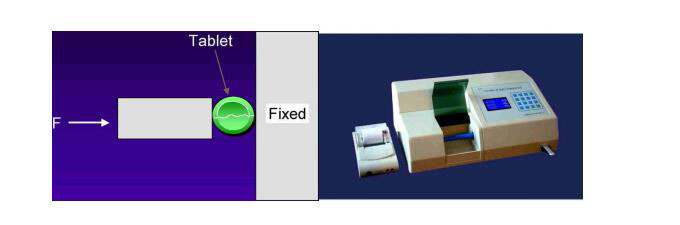
11) Hardness tester
a. Mansanto hardness tester
b. Four-purpose tablet tester
c. Qualified tablets can endure a force of 30-40N
12) Tablet friability testing
a. Total weight: 6.5g;
b. Rotating for 100 times;
c. Weight loss cannot be over 1%.
13) disintegration test
a. Ordinary tablets: disintegrating in 15min;
b. Sugar-coated tablets: disintegrating in 1h;
c. Film-coated tablets: disintegrating in 0.5h;
d. Enteric coated tablets: no crack, disintegration or softening in hydrochloric acid for 2h, while completely disintegrating in 1h in phosphate buffer (pH=6.8);
e. Buccal tablets: not disintegrating in 10min;
f. Sublingual tablets: 5min;
g. Soluble tablets: 3min;
h. Effervescent tablets: 5min;
i. Hard capsules;
j. Soft capsules;
k. Dripping pill.
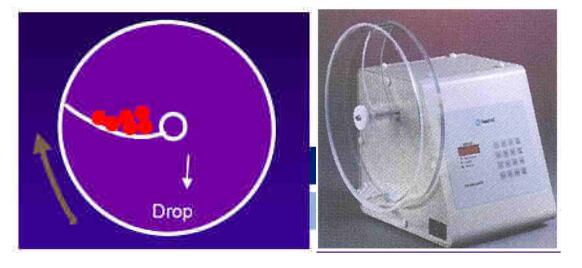
14) Dissolution rate or release rate
a. Definition: the speed and degree of medicine dissolution in regulated medium.
b. Dissolution medium:
a) Pure water;
b) Artificial intestinal fluid (phosphate buffer, pH=6.8)
c) Artificial gastric juice (hydrochloric acid buffer, pH=1.0)
d) Big cup: 900ml; small cup: 200ml;
e) Rotating speed: 50rpm or 100rpm;
f) Temperature: 37±0.5°C
c. Significance of dissolution testing
a) Dissolution rate: ordinary preparation;
b) Release degree: sustained and controlled release preparation;
c) To establish relevant indicators;
d) E.g. dissolution rate of ketoconazole capsules in 30min should be 80% of its content.
9. Tablet package and storage
1) Multiple dose package
a. Glass bottle (tube);
b. Plastic bottle (box);
c. Soft plastic sachet
2) Monodose package
a. Blister
b. Strip
3) Example 1
a. Stable and compressible tablet
b. Paediatric Compound Sulfamethoxazole Tablets
c. Granulation method: wet granulation and tablet press