What is Powder & Granule I
Writer: admin Time:2020-05-23 13:56 Browse:℃
1. Solid preparation
1) Commonality
a. Better physical and chemical stability, lower cost and being carried or taken conveniently;
b. The same unit process (crushing, screening, mixing, etc.) before preparation.
c. Medicine dissolve in body first, and then can pass through physiological membrane and be absorbed into blood.
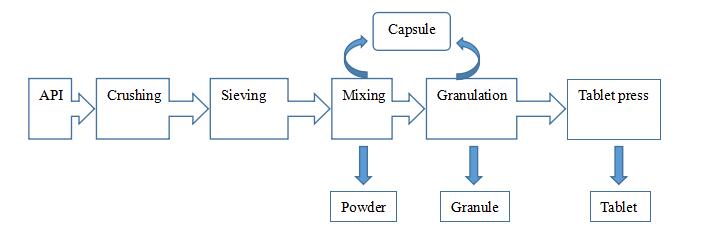
2) Flow chart
3) Absorption pathway
Taking medicine - disintegration - dissolution - (physiological membrane) - blood circulation
4) Noyes-Whitney dissolution equation
dC/dt: dissolution speed
k: dissolution speed constant
S: dissolution area
Cs: medicine dissolution rate
D: expanding coefficient
h: expanding layer thickness
When Cs>>C, the equation can be simplified to:

5) Measures to better API dissolution speed
The dissolution speed is proportional to S, Cs and k
a. To increasing medicine dissolution area (S): powdering medicine or narrowing medicine size;
b. To increasing medicine dissolution rate (Cs): changing crystal or preparing solid dispersion;
c. To increasing dissolution rate constant (k): mixing more, reducing viscosity or increasing temperature.
2. Powders
1) Definition
Powders refers to the a dry powdery preparation which is made by API and appropriate excipients after crushing and uniformly mixing.
2) Classification
a. According to application:
a) Oral powders: being taken after dissolution in water or directly;
b) Topical powders: skin, oral cavity, throat, cavity;
b. According to medicinal: single powders and composite powder;
c. According to dose: divided powders and undivided powders
3) Features
a. High crushing degree, large specific surface area, quick dispersion and quick result;
b. Large tropical area for protection and astringency;
c. Convenient storage, transport and carrying;
d. Simple preparation techniques, divided dose and convenient taking for infants and babies.
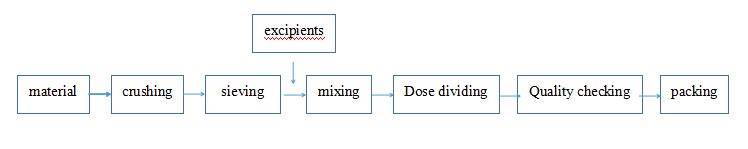
4) Preparation
3. Crushing
1) Definition
Crushing refers to the operation in which large solid material is broken into granules of fine powders of proper size by mechanical force.
2) Goals
a. Increase surface area for higher insoluble medicine’s dissolution speed and bioavailability;
b. Narrow size distribution for uniform mixing;
c. Increase granule amount for higher dispersion;
d. For extraction of natural herbs’ active ingredients.
3) Method
a. Dry crushing: materials are crushed while being dry (moisture content<5%)
b. Wet crushing: materials are crushed after proper amount of water or liquid is added. It is also called liquid crushing.
a) For medicines difficult to be crushed, such as camphor, borneol, menthol...
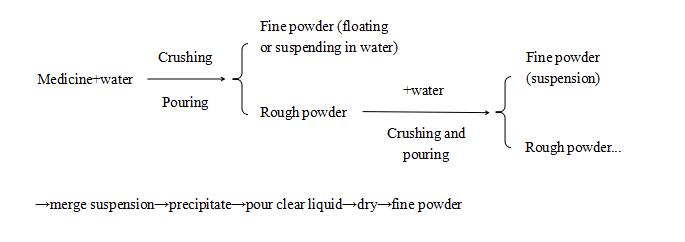
b) For fine insoluble medicines, such as calamine, vermillion, realgar, pearl, talc... can be prepared by washing paddling process:
4) Crushing machine
a. Ball mill: simple structure, working in an enclosed condition, little dust... It can be used for highly toxic and precious medicine or medicine of strong hygroscopicity. Besides, the product can be bacteria-free. If operated properly, the machine can produce 10μm powder.
b. Impact crusher
a) It is known as universal crusher. Its force is mainly impact;
b) It can be applied to crush brittle or resilient materials to medium-fine , fine or ultra-fine powder.
c. Jet mill
a) It is used to crush materials to size of 3-20μm.
b) High pressure nozzle adopts Joule-Thomson cooling effect, being applicable for materials of temperature sensitivity or low melting point.
c) The equipment is simple and can be used to prepare bacteria-free powders.
d) Crushing cost is pretty high.
4. Sieving
1) Definition:
To separate materials of different size so that granules of uniform size can be got.
2) Meanings:
a. Quality control: Pharmacopoeia has requirements on granularity of powders and granules;
b. Production control: obvious influence on mixing degree, fluidity, filling, hardness and tablet breaking during mixing, granulation and tablet press.
3) Screen and sieving equipment


Shaking screen
4) Screen specification table regulated in Chinese Pharmacopoeia
|
No. |
Mesh diameter (average value) |
Meshes |
|
#1 |
2000μm ±70μm |
10 |
|
#2 |
850μm ±29μm |
24 |
|
#3 |
355μm ±13μm |
50 |
|
#4 |
250μm ±9.9μm |
65 |
|
#5 |
180μm ±7.6μm |
80 |
|
#6 |
150μm ±6.6μm |
100 |
|
#7 |
125μm ±5.8μm |
120 |
|
#8 |
90μm ±4.6μm |
150 |
|
#9 |
75μm ±4.1μm |
200 |
Definition of mesh: the quantity of screen hole in a length of one inch (2.54cm).
5) Powder classification
|
Solid powder grade |
The screen that powder pass through completely |
The screen that powder can pass through |
Proportion |
|
Coarsest powder |
#1 |
#3 |
≤20% |
|
Coarse powder |
#2 |
#4 |
≤40% |
|
Medium powder |
#4 |
#5 |
≤60% |
|
Fine powder |
#5 |
#6 |
≥95% |
|
Finest powder |
#6 |
#7 |
≥95% |
|
Ultra-fine powder |
#8 |
#9 |
≥95% |
6) Fineness requirements on powders
a. General powders can pass through #6 screen (fine powder, 100 meshes, 150μm);
b. Insoluble medicine, astringent, absorbent, pediatric or externally used powders can pass through #7 screen (the finest powder, 120 meshes, 125μm);
c. Powdery suspending medicine in eye ointment should pass through 9# screen (ultra-fine powder: 200 meshes, 75μm).